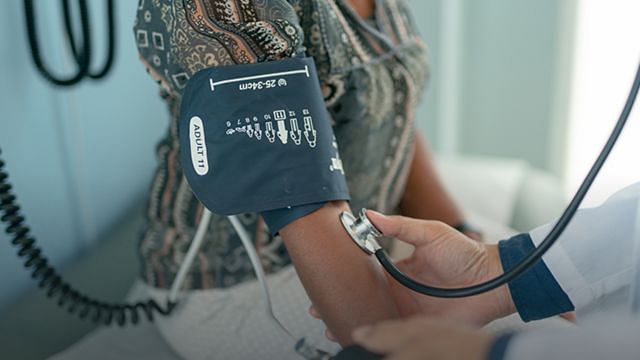
FDA Approves Novel Oral Antihypertensive for Resistant Hypertension
21 Mar 2024 • The US FDA has recently granted approval for aprocitentan as a treatment for lowering blood pressure. It is indicated for adults with treatment-resistant hypertension, to be used in combination with other antihypertensive medications.
This is the first oral antihypertensive employing a novel therapeutic pathway to be approved in nearly 40 years. The endothelin receptor antagonist targets endothelin-1 (ET-1) receptors ETA and ETB, thereby inhibiting their binding with ET-1. Endothelin's effects align closely with hypertension pathophysiology, making it a significant contributor to elevated BP and a major driver of aldosterone production.
The recommended daily dose is 12.5 mg, taken orally once a day, with or without food.
Source: FDA | Read full story